What is Periodic Table?
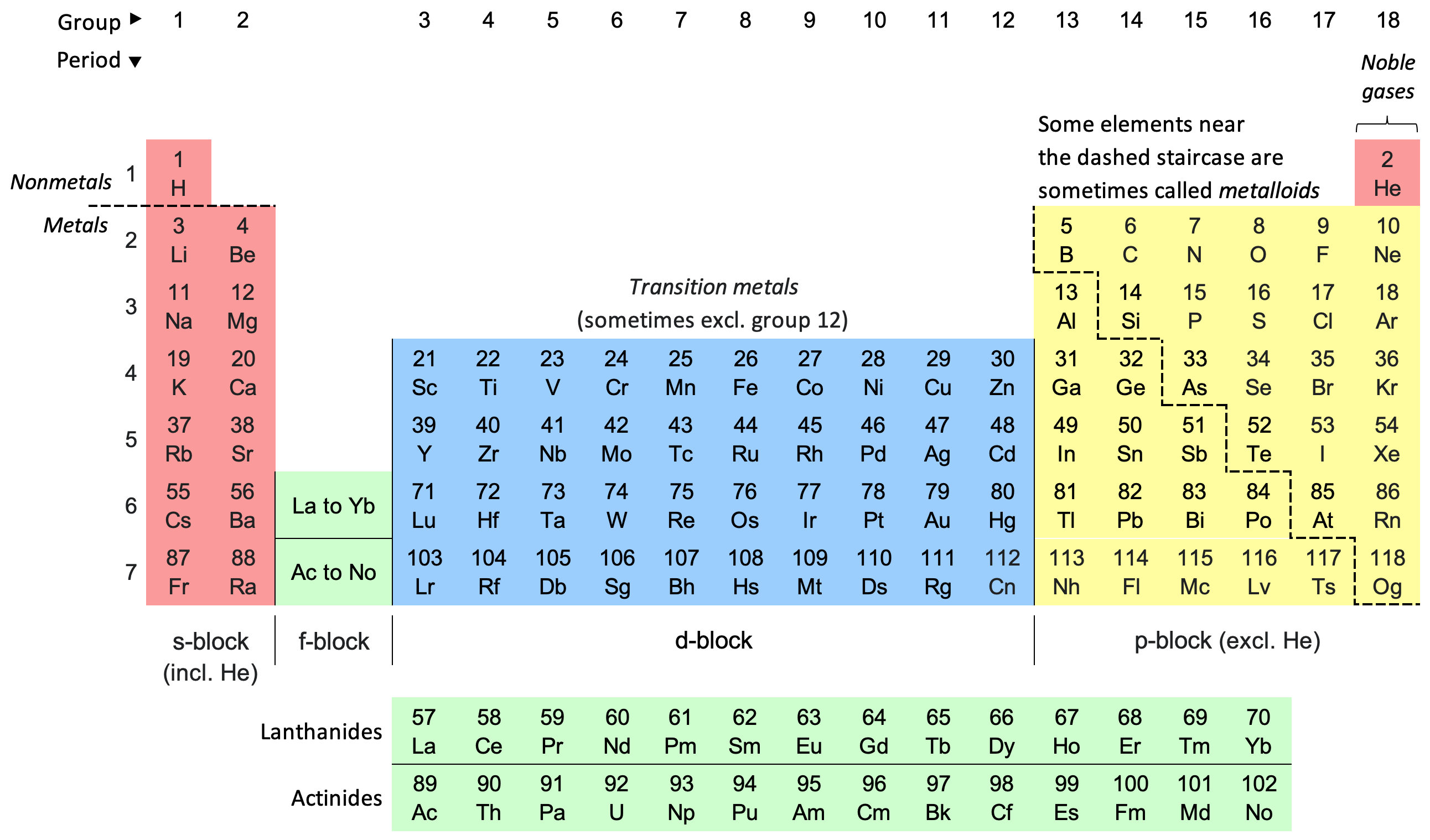
The periodic table is a way to list the elements. The elements are listed in the table according to their atomic structure. This includes the number of protons they have as well as the number of electrons they have in their outer shell. From left to right and top to bottom, the elements are listed in order of atomic number, which is the number of protons in each atom.
Why is it called the Periodic Table?
It is called “periodic” because the elements are ordered by period or period. From left to right, the elements are lined up by atomic number (the number of protons in their nucleus). Some columns are omitted so that elements with the same number of valence electrons line up on the same column. When aligned this way, the column elements have similar properties.
Groups
Groups are columns of the periodic table. There are 18 columns or groups and different groups have different properties.
An example of a group is a noble gas or an inert gas. All of these elements line up in the eighteenth or last column of the periodic table. They all have an outer shell filled with electrons, which makes them very stable (they tend not to react with other elements). Another example is the alkali metals lined up in the leftmost column. They are all very similar in that they have only one electron in their outer shell and are very reactive. You can see all the groups in the table below.
This association and grouping of similar elements helps chemists when working with elements. They can understand and predict how an item might react or behave in a given situation.
Element Abbreviations
Each element has its own name and abbreviation in the periodic table. Some abbreviations are easy to remember, such as H for hydrogen. Some are a bit harder like Fe for iron or Au for gold. As for gold, the word “Au” comes from the Latin word for gold “aurum”.
Who Invented it?
The periodic table was proposed by Russian chemist Dmitri Mendeleev in 1869. Using the table, Mendeleev was able to accurately predict the properties of many elements before they were actually discovered.
Interesting facts about the Periodic Table
Carbon is unique in that it is known to form up to 10 million different compounds. Carbon is important to the existence of life.
Francium is the rarest element on earth. There are probably no more than a few ounces of it on earth at any given time.
The only letter not in the periodic table is the letter J.
The country Argentina is named after the element silver (symbol Ag) which is argentum in Latin.
Although there is helium on Earth, it was first discovered by observing the sun.